Allergy Clinical Trial Services Market: Technology Evolution, Regional Demand & Forecast Scenarios to 2036
Advancing allergy research through specialized clinical trial services supporting safe, effective, and innovative allergy treatment development worldwide.
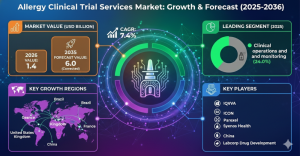
ROCKVILLE, MD, UNITED STATES, February 12, 2026 /EINPresswire.com/ -- The global allergy clinical trial services market is on a path toward significant expansion, with its valuation expected to rise from USD 1.4 billion in 2026 to USD 2.9 billion by 2036. This growth, representing a 7.4% CAGR, is fueled by a structural shift in allergology from symptomatic management to disease-modifying precision immunotherapies. As understanding of hypersensitivity mechanisms deepens, pharmaceutical pipelines are increasingly prioritizing clinically validated desensitization systems over traditional antihistamine treatments.Request for Sample Report | Customize Report | Purchase Full Report - https://www.factmr.com/connectus/sample?flag=S&rep_id=14125
Market snapshot: global Allergy Clinical Trial Services Market demand 2026 - 2036
Market size 2026? The market is estimated at USD 1.4 billion.
Market size 2036? The industry is projected to reach USD 2.9 billion.
CAGR? The market will expand at a 7.4% CAGR (2026–2036).
Leading product segment(s)? Clinical operations and monitoring and Allergic rhinitis both hold leading shares of 24.0% each within their respective categories.
Leading material type? In terms of trial focus, Allergic rhinitis establishes dominance due to high disease prevalence.
Leading end use? While specific shares were not provided, Pharma and Biotech sponsors are primary end users driving trial initiations.
Key growth regions? China (14.7% CAGR), India (13.1%), Brazil (11.5%), and the United States (9.9%).
Top companies? IQVIA, ICON, Parexel, Syneos Health, and Labcorp Drug Development.
Market Momentum
The allergy clinical trial services market is characterized by steady value appreciation. Valued at USD 1.4 billion in 2026, the market is forecast to grow to USD 2.9 billion by 2036. Key valuation milestones throughout the decade include the following points:
2026: USD 1.4 billion
2028: (Steady upward gain)
2030: (Consistent growth)
2031: (Expansion phase)
2033: (Mature expansion)
2036: USD 2.9 billion
Why the Market is Growing
The industry is expanding due to a rising pediatric food allergy prevalence that has shifted focus toward active desensitization protocols. Regulatory innovations, such as needle-free delivery systems (e.g., neffy nasal spray), are democratizing access to emergency treatments. Furthermore, the move toward precision immunotherapy requires complex trial designs that necessitate specialized CRO services to manage intensive real-time oversight and safety protocols for hypersensitivity manifestations.
Segment Spotlight
1) Product Type (Clinical Operations & Monitoring)
Accounting for a 24.0% share, clinical operations and monitoring lead because allergic reactions require intensive oversight. Allergic manifestations can rapidly escalate to anaphylaxis, necessitating on-site medical personnel during allergen challenges. CROs are establishing premium pricing by deploying safety infrastructure that exceeds general therapeutic requirements.
2) Trial Focus (Allergic Rhinitis)
Allergic rhinitis captures 24.0% of trial focus because its high prevalence supports large-scale efficacy trials. Unlike food allergy trials, seasonal rhinitis allows for natural allergen challenges during pollen seasons, which reduces protocol complexity while maintaining the clinical relevance required for pharmaceutical investment.
3) End Use (Pharma & Biotech Sponsors)
Trial initiations throughout the forecast period reflect a strategic emphasis on combination immunotherapy protocols. Sponsors are increasingly investing in oral tolerance induction synergized with biologic interventions, creating a high demand for specialized service providers capable of managing these complex immunology frameworks.
Drivers, Opportunities, Trends, Challenges
Drivers: The intensification of pediatric food allergy research is moving beyond avoidance toward active desensitization. This is supported by regulatory innovation in delivery systems and elevated public understanding of multi-allergen sensitization patterns, creating a surge in demand for transformative clinical interventions.
Opportunities: Component-Resolved Diagnostic Integration presents a significant opportunity. CROs that incorporate molecular allergen identification matching individual sensitization patterns can differentiate themselves by offeringMechanistic patient stratification, supporting personalized protocol adaptations for premium trial segments.
Trends: The use of Artificial Intelligence (AI) in patient recruitment is a major trend. Machine learning algorithms analyzing electronic health records can reduce enrollment timelines by nearly 30%. This allows for the identification of complex allergic phenotypes through natural language processing of clinical documentation.
Challenges: Patient recruitment remains a critical bottleneck. Geographic variation in allergen patterns and intense competition for pediatric subjects can lead to trial extensions or failures. Additionally, the complexity of validating mechanistic biomarkers like IgE/IgG4 ratios limits innovation adoption beyond traditional symptom scores.
Country Growth Outlook (CAGR)
The global allergy clinical trial services market is set for varied regional growth through 2036, led by China (14.7% CAGR) and India (13.1%) due to revamped regulatory pathways. Brazil (11.5%) and the USA (9.9%) follow, driven by streamlined approvals and a focus on novel emergency treatments. In Europe, growth is more specialized, with the UK (8.3%) benefiting from fast-track assessments, while Germany (5.1%) and France (3.5%) maintain steady expansion supported by established regulatory ordinances and academic expertise.
Competitive Landscape
The allergy clinical trial services market is led by IQVIA, which utilizes integrated technology platforms and bioanalytical laboratories for mechanistic research. ICON competes through specialized pediatric research units, particularly for food allergy and atopic dermatitis. Other dominant players include Parexel, Syneos Health, and Labcorp Drug Development, all of whom are focusing on specialized immunology safety infrastructure and real-world evidence generation to support long-term immunotherapy outcomes.
FAQ
Why is clinical monitoring the leading service line?
Allergic reactions can escalate to anaphylaxis rapidly, requiring labor-intensive, real-time medical oversight during allergen exposure challenges and oral immunotherapy.
How is China accelerating its allergy trial market?
The NMPA’s 30-day IND pathway has reduced clinical startup timelines by 50%, making the region highly attractive for globally synchronized trials.
What is the significance of the DBV Technologies Phase 3 trial?
The VITESSE trial evaluates Viaskin Peanut, a skin patch immunotherapy that represents an alternative to oral treatments by avoiding gastrointestinal side effects.
To View Related Report:
Heart Failure Monitoring Systems Market https://www.factmr.com/report/1088/heart-failure-monitoring-systems-market
Dental Curing Lights Market https://www.factmr.com/report/1095/dental-curing-lights-market
Tele-ICU Services Market https://www.factmr.com/report/1100/tele-icu-services-market
Microwave Imaging Market https://www.factmr.com/report/1102/microwave-imaging-market
About Fact.MR
Fact.MR is a global market research and consulting firm, trusted by Fortune 500 companies and emerging businesses for reliable insights and strategic intelligence. With a presence across the U.S., UK, India, and Dubai, we deliver data-driven research and tailored consulting solutions across 30+ industries and 1,000+ markets. Backed by deep expertise and advanced analytics, Fact.MR helps organizations uncover opportunities, reduce risks, and make informed decisions for sustainable growth.
S. N. Jha
Fact.MR
+ +1 628-251-1583
sales@factmr.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.